Statin reduces the risk of cardiovascular disease in people living with HIV
REPRIEVE trial demonstrated that daily pitavastatin reduced the risk of cardiovascular disease in people living with HIV by 35%.
About REPRIEVE Trial
REPRIEVE is the first-ever randomized clinical trial to determine whether statin use prevents atherosclerotic cardiovascular disease events in persons with HIV infection who are at low-to-moderate risk for cardiovascular events.1
The investigators used pitavastatin calcium as the statin in this study because it does not interact with the drugs that are used in antiretroviral therapy.
This information is intended for Healthcare Providers. Please note the limitation of use statement in both the Livalo (pitavastatin calcium) and Zypitamag (pitavastatin magnesium) Package Inserts. The effect of Pitavastatin on cardiovascular morbidity and mortality has not been determined.
HIV, Cardiovascular Disease, and Statins
People living with HIV infection are living longer and longer thanks to advancements in medications to manage HIV infection. As a result, this patient population is now living long enough to experience non-AIDS-defining illnesses and, notably, cardiovascular disease.
People with HIV are at a much greater risk for cardiovascular disease than the general population.
The risk of myocardial infarction in people with HIV is 1.5–2.0 times higher, and dyslipidemia has been reported in up to 80%. Possible mechanisms for this include a combination of chronic inflammation and dysregulation of the immune system.
Mortality from cardiovascular disease has increased substantially from 1999 to 2013 for people living with HIV while this number has consistently gone down for the general population.2

Adapted from Figure 3 in Feinstein MJ, et al. Am J Cardiol. 2016;115(12):1760-6.
Statin Use In People With HIV
Treating dyslipidemia has historically been challenging for this patient population given the increased potential for drug interactions due to competing cytochrome P450 (CYP450) metabolism with statins and antiretroviral therapies. These interactions can lead to issues with efficacy and statin tolerability.
There is also a lack of data in this patient population and lack of guideline recommendations, leaving prescribers without great guidance on how to manage this patient population.
Data analyzed from the National Ambulatory Medical Care Survey from 2006 to 2013 identified that physicians generally underused guideline-recommend cardiovascular care in people living with HIV.3

Adapted from Figure 1 of Ladapo JA et al. J Am Heart Assoc. 2017;6:e007107.
This data may explain higher rates of adverse cardiovascular events among patients with HIV in the U.S., given that they are being untreated compared to the general patient population.
Improved Access to Pitavastatin through Marley Drug
Pitavastatin is available under the brand name Livalo (pitavastatin calcium) and Zypitamag (pitavastatin magnesium). The FDA considers these medications to be bioequivalent.
Marley Drug pharmacy offers Zypitamag (Pitavastatin) for $1.15/day or $34.50/month. The first 30 days are free for new patients to allow them to try the medication and ensure they can tolerate it.
Zypitamag is also available on certain AIDS Drug Assistance Program (ADAP) state formularies. Find more information here.
* Conditions apply. Visit Zypitamag.com for more information.
† Livalo® pricing based on Average Wholesale Price.
Statin Drug Interactions
Most statins are processed by the CYP450 family of enzymes, which can lead to drug interactions when paired with antiretroviral therapy.
Two statins, Pitavastatin and pravastatin, are only minimally processed by the CYP450 pathway and are reasonable treatment options for those living with HIV who have high cholesterol.

How pitavastatin and pravastatin are processed by our bodies
The principal route of pitavastatin metabolism is glucuronidation via liver uridine 5'-diphosphate glucuronosyltransferase (UGT) with subsequent formation of pitavastatin lactone. There is only minimal metabolism by the cytochrome P450 system. Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. Pravastatin is processed by CYP3A4 (part of CYP450), but unlike other statins it’s not extensively metabolized by this enzyme. Instead, it’s mostly excreted unchanged in the bile. Because CYP450 isn’t significantly involved in pravastatin’s metabolism, pravastatin also has a decreased risk of drug interactions.
Due to these interactions, and previous randomized trials, which demonstrated pitavastatin was clinically superior to pravastatin in people living with HIV (see data below), pitavastatin was the statin of choice for the REPRIEVE trial.
Pitavastatin Clinically Superior to Pravastatin in those with HIV4
The INTREPID trial was a randomized, double-blind, active-controlled, phase 4 trial which recruited adults aged 18–70 years with controlled HIV who were on antiretroviral therapy for at least six months and dyslipidemia.
Patients were randomized to receive pitavastatin 4mg (n=126) or pravastatin 40mg (n=126) once daily for 12 weeks, followed by a 40-week safety extension.
At 12 weeks, LDL-cholesterol was reduced by 31.1% with pitavastatin and 20.9% with pravastatin.
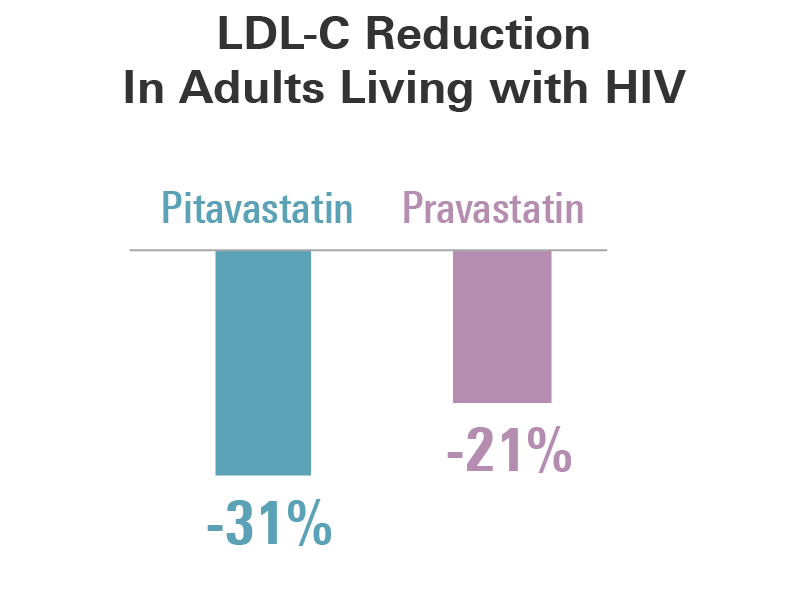
Mean percent changes at week 12
| Pitavastatin 4 mg |
Pravastatin 40 mg |
|
| ApoB | -26% | -19% |
| Triglycerides | -3% | -4% |
| HDL-C | +5% | +6% |
| hsCRP | -12% | 0% |
This study excluded patients being treated with darunavir, or who had homozygous familial hypercholesterolemia, or any condition causing secondary dyslipidemia, or a history of statin intolerance, diabetes, or coronary artery disease.
Both treatments have a neutral effect on glucose metabolism parameters.
Pitavastatin is as Accessible as a Generic Statin Through Marley Drug
Your patients can access Zypitamag (Pitavastatin) throughout the pharmacy without needing insurance. That means:
- No prior authorizations
- No step therapy
- No insurance hassles
One consistent price. Just $1.15/day.
Send prescriptions to Marley Drug, and your patients will receive the first 30 days for Zypitamag free.
REPRIEVE Trial Design
The REPRIEVE trial randomized 7769 participants who were living with HIV infection on stable antiretroviral therapy (ART) at low-to-moderate risk of cardiovascular disease to pitavastatin 4 mg or placebo (for up to 8 years).
The purpose of the REPRIEVE trial was to determine whether statin use prevents atherosclerotic cardiovascular disease events in persons with HIV infection who are at low-to-moderate risk for cardiovascular events.
| Asymptomatic people with HIV on ART with 10-year ASCVD risk ≤15% |
| Placebo vs. Pitavastatin 4 mg/day |
| Primary Endpoint: MACE (CV Death, MI, Unstable Angina, TIA/Stroke, Revascularization, PAD) |
Secondary Endpoints:
|
For the primary endpoint, MACE was defined as cardiovascular (CV) death, myocardial infarction (MI), hospitalization for unstable angina, stroke, transient ischemic attack (TIA), peripheral arterial ischemia (PAD), revascularization, or death from an undetermined cause.
The study excluded anyone who took a statin in the last 90 days, and those with known atherosclerotic cardiovascular disease.
REPRIEVE Trial Results
Daily pitavastatin reduced the risk of cardiovascular disease in people living with HIV by 35%.
The pitavastatin group had a significantly lower incidence of major adverse cardiovascular events than those in the placebo group, with a hazard reduction of 35% over a median of 5.1 years of follow-up.

Treatment with pitavastatin was consistent across major subgroups, including men and women, and across different global regions. In addition, there were no differences in groups based on CD4 count levels or duration of ART therapy.
Safety Events
The incidence of nonfatal serious adverse events was similar in both groups. There was a higher rate of diabetes mellitus in the pitavastatin group. There was no apparent treatment effect on glucose levels.
A higher number of muscle-related symptoms were reported in the pitavastatin group. However, a very small portion of patients discontinued their medication (1.1% in the pitavastatin group vs. 0.5% in the placebo group).
| Event | Pitavastatin (N=3888) | Placebo (N=3881) | Indidence Rate Ratio (95% CI) |
||
| No. With Event | Incidence Rate (No/100 person-yr) |
No. With Event | Incidence Rate (No/100 person-yr) |
||
| Nonfatal serious advers event | 695 | 4.16 | 694 | 1.01 | 1.01 |
| Diabetes mellitus | 206 | 1.13 | 155 | 0.84 | 1.35 |
| Myalgia, or myopathy of grade ≥3 or treatment limiting | 91 | 0.49 | 53 | 0.28 | 1.74 |
| Rhabdomyolsis of grade ≥ 3 or treatment limiting | 3 | 0.02 | 4 | 0.02 | 0.75 |
| Alanine aminotransferase elevation of grade ≥3 | 11 | 0.06 | 8 | 0.04 | 1.38 |
| Any adverse event | 1304 | 8.88 | 1256 | 8.37 | 1.06 |
Key Takeaways of REPRIEVE Trial
- People living with HIV have elevated risks of cardiovascular disease
- Despite HIV being considered a risk equivalent, no prior trial has assessed a primary prevention strategy for this group who would not typically be recommended for statin therapy
- Taking a daily statin can lower the risk by more than one-third. Among persons living with HIV 40-75 years of age, on ART, with low-to-moderate risk, and normal range LDL, treatment with pitavastatin is effective and prevents major adverse cardiovascular events.
- Considerations should be given to expanding treatment guidelines in this regard
Get the First 30-days of Pitavastatin FREE through Marley Drug
We offer patients the first 30 days of Zypitamag (pitavastatin) free with a valid prescription.
Medications are shipped via USPS First Class Mail and arrive in 2-3 business days.
Please note the limitation of use statement in both the Livalo (pitavastatin calcium) and Zypitamag (pitavastatin magnesium) Package Inserts. The effect of pitavastatin on cardiovascular morbidity and mortality has not been determined.